Over time, high tunnels tend to accumulate salts, which can drive up the alkalinity and pH of the soil. Acidifying irrigation water is one way to address this problem, and it can be done with varying degrees of investment and technology.
When salts build up in the soil, the alkalinity of our soil also increases. Alkalinity refers to the ability of water to neutralize acids and bases, and thus maintain a stable pH. Over time, this can lead to an increase in the pH of soil in high tunnels. This is problematic, because essential plant nutrients become less available when the soil pH moves above 7. Even if the pH of the soil is within the optimal range, irrigation water with a high pH can lead to decreased nutrient uptake, especially in tunnels.
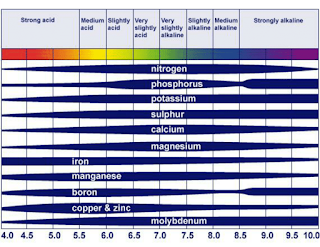
The widely used "pH Nutrient Availability Chart" shows how the availability of nutrients changes according to the pH. Image from Roques et al., 2013
We often see this process start to happen in year 3 or 4 after installing a tunnel, especially in tunnels where heavy loads of compost have been applied. Your plants might look amazing immediately after a heavy compost application, but over time as the pH creeps up, the higher pH can inhibit nutrient uptake. Sometimes this means that while the soil has very concentrated levels of nutrients, plants may still exhibit deficiency symptoms. Adding more nutrients will not fix the problem; rather, growers in this situation need to focus on bringing the pH back down and reducing the salt concentrations in the soil. Some plants are more tolerant to high salt levels than others; review the table below for a comparison of vegetables and their tolerance to salts.
Low-tech solutions to manage salts and pH
There are some simple ways to reduce salt build-up in your high tunnel soil:
Author: Natalie Hoidal, UMN Extension Educator, Local Foods and Vegetable Crops
Reviewed by Dr. Paulo Pagliari and Dr. Vasudha Sharma
Understanding the problem
When we apply fertilizer, compost, and manure to our soil, we are often adding salts. Calcium, magnesium, potassium, sodium, and ammonium are all examples of salts that we commonly add to agricultural soils. Typically, acidic rain water washes these salts out of the soil to keep things balanced. However, in tunnels where we lack access to rain, this washing out process does not happen. Further, sometimes the groundwater we use to irrigate in tunnels is also rich in salts.When salts build up in the soil, the alkalinity of our soil also increases. Alkalinity refers to the ability of water to neutralize acids and bases, and thus maintain a stable pH. Over time, this can lead to an increase in the pH of soil in high tunnels. This is problematic, because essential plant nutrients become less available when the soil pH moves above 7. Even if the pH of the soil is within the optimal range, irrigation water with a high pH can lead to decreased nutrient uptake, especially in tunnels.
The widely used "pH Nutrient Availability Chart" shows how the availability of nutrients changes according to the pH. Image from Roques et al., 2013
We often see this process start to happen in year 3 or 4 after installing a tunnel, especially in tunnels where heavy loads of compost have been applied. Your plants might look amazing immediately after a heavy compost application, but over time as the pH creeps up, the higher pH can inhibit nutrient uptake. Sometimes this means that while the soil has very concentrated levels of nutrients, plants may still exhibit deficiency symptoms. Adding more nutrients will not fix the problem; rather, growers in this situation need to focus on bringing the pH back down and reducing the salt concentrations in the soil. Some plants are more tolerant to high salt levels than others; review the table below for a comparison of vegetables and their tolerance to salts.
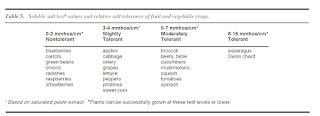 |
| Table from UMN Nutrient Management Guide for Commercial Fruit and Vegetable Growers. Click for higher resolution. |
Low-tech solutions to manage salts and pH
There are some simple ways to reduce salt build-up in your high tunnel soil: - Be careful about over-applying compost. While tunnels are high value environments and it’s tempting to add tons of compost, this can have negative effects on salinity in the long-term. Test your compost to make sure the salt levels (measured in electrical conductivity, but also check calcium, magnesium, potassium) are not excessive.
- In years when you plan to replace the plastic, remove it in late summer and add the new plastic the following spring. The rain and snow should help to leach out some of the salts in the soil.
- Some growers with access to bobcat-type machinery will shovel large quantities of snow into their tunnels in the winter
- You can also flood your tunnel with irrigation water periodically if your water source is not very hard. For growers with very alkaline water, or high pH water, this process should either be done with water from elsewhere, or water that has been acidified.
Acidifying your irrigation water
Using acidified water can help to mitigate pH issues in tunnels. Some growers choose to bring the pH of their irrigation water down to 5.8 to mimic rain water. Keep in mind that if you are using drip irrigation, pH may still be high between rows. One option to address this is to use a sprinkler system to flood the entire tunnel between crops, several times throughout the season. Another option is to continuously acidify your water.Materials
For organic growers, the only approved acid for this purpose is citric acid. Conventional growers can also use sulfuric acid, nitric acid, and phosphoric acid. These acids tend to be more concentrated than citric acid, meaning you can add less of them to get the same effect, but they can also be more dangerous and should be handled with care. It’s also important to keep in mind that sulfuric acid adds sulfur, nitric acid adds nitrogen, and phosphoric acid adds phosphorus.How much acid to add?
There are a few approaches you can take to calculate how much acid is needed to bring your water pH to a certain level.Approach 1: Take a water sample to determine your pH and alkalinity. While you can easily test your pH yourself with strips, alkalinity is harder to measure. You can purchase a meter, or simply send your water to a lab for about $10 and they can do the sample.
Once you know your alkalinity, you can use the Alk Calc tool to figure out how much acid you’ll need to add per volume of water (e.g. fl oz. per 100 gallons) to reach your desired pH. Unfortunately, this tool does not provide numbers for citric acid.
Once you know your alkalinity, you can use the Alk Calc tool to figure out how much acid you’ll need to add per volume of water (e.g. fl oz. per 100 gallons) to reach your desired pH. Unfortunately, this tool does not provide numbers for citric acid.
Approach 2: You can also take a DIY approach. Start with a known volume of water, such as 50 gallons. Add small amounts of your acid to the water, mix, and test with a pH strip. Do this repeatedly until you notice your pH start to change, and then continue until you reach the desired pH. Keep in mind that it may take a long time to observe a change, but once it happens, it can happen quite quickly, so frequent measurements are important.
Equipment
To acidify your water, you’ll need some kind of injector. Our Irrigation Set-ups for Specialty Crop Growers page has an overview of types of injectors at the end of the page.- If you have a continuously operating injector, such as a volumetric proportioning injector (e.g. a Dosatron), you can have a continuous flow of a precise amount of acidified irrigation water every time you irrigate.
- If you have an EZ-Flo type injector, you'll have a couple of injection settings like 1:100 or 1:1000. Your manual will tell you what these represent. For example, with one model of EZ Flo, 1000:1 is equivalent to 2/3 tsp product per gallon of water flowing through, 500:1 if equivalent to 1 1/2 tsp per gallon, and 100:1 is equivalent to 2 tbsp per gallon. If you know how much acid you need per gallon to reach your desired pH, you can choose the right setting accordingly.
- If you have a simpler system like a venturi-style injector, your process will be a bit less precise. If you know how much water comes of your irrigation system in an hour, you can figure out how much you’ll be watering in a given day, and then inject the corresponding amount of acid.
Dig deeper
Two great resources that provide much more in-depth content about acidifying irrigation water are:- https://greenhouse.hosted.uark.edu/Unit09/Section03.html
- https://ag.umass.edu/greenhouse-floriculture/fact-sheets/adjusting-alkalinity-with-acids
Author: Natalie Hoidal, UMN Extension Educator, Local Foods and Vegetable Crops
Reviewed by Dr. Paulo Pagliari and Dr. Vasudha Sharma
*Re-posted and updated from 2022
Comments
Post a Comment